Revista Paraguaya de Reumatología, Vol. 1, Nº 1, 2015
ISSN 2413-4341
ORIGINAL
ANTIPHOSPHATIDYLSERINE ANTIBODIES IN PATIENTS WITH PRIMARY ANTIPHOSPHOLIPID SYNDROME AND IN HEALTHY INDIVIDUALS
Alexandre Wagner Silva de Souza1,2, Alessandra Dellavance2, Jéssica Martins Camargo2, Sonia R S Siciliano2, Emilia Inoue Sato1, Luis Eduardo Coelho Andrade1,2
Rheumatology Division, Universidade Federal de São Paulo - Escola Paulista de Medicina (Unifesp-EPM), São Paulo, Brazil
Fleury Group, Research and Development, São Paulo, Brazil
Corresponding author: Email: luis.andrade@unifesp.br (L. E. Coelho Andrade)
ABSTRACT
Objective: To investigate the prevalence of IgM, IgG and IgA anti-phosphatidylserine (aPS) antibodies in patients with primary antiphospholipid syndrome (PAPS) and in healthy controls; to analyze sensitivity, and specificity of aPS antibodies for the diagnosis of APS and finally to assess associations between aPS antibodies with specific APS manifestations.
Methods: A cross-sectional study was performed in 36 female PAPS patients and in 200 blood donors. IgM, IgG, and IgA antiphosphatidylserine (aPS) antibodies were tested in PAPS patients and controls using an in house technique and a commercial kit. PAPS patients were also tested for lupus anticoagulant (LAC), IgM and IgG anticardiolipin (aCL) antibodies, and for anti-β2 glycoprotein I (anti-β2GPI) antibodies.
Results: The prevalence of IgM, IgG, and IgA aPS antibodies in PAPS patients was as follows: 10.8-16.7%, 32.4-35.7%, and 16.1%, respectively. Although a relatively low sensitivity was found for aPS antibodies in PAPS, the specificity of IgM, IgG, and IgA aPS antibodies for PAPS was 94.7-98.9%, 95.3-96.3%, and 97.9%, respectively. All aPS isotypes were significantly associated with obstetric manifestations of APS. IgM aPS antibodies were associated with an increased risk of venous and arterial thrombosis. IgA aPS antibodies were associated with arterial thrombosis whereas IgG aPS antibodies were associated with an increased risk of venous thrombotic events. IgM and IgG aPS antibodies were frequently found in association with anti-β2GPI antibodies.
Conclusions: The prevalence of aPS antibodies is low in PAPS but these antibodies are highly specific for PAPS and are associated with specific PAPS manifestations.
Keywords: Antiphospholipid Syndrome, Antiphospholipid Antibodies, Antiphosphatidylserine Antibodies, Thrombosis, Autoantibodies
ANTICUERPOS ANTIFOSFATIDILSERINA EN PACIENTES CON SÍNDROME ANTI FOSFOLIPIDO PRIMARIO Y EN INDIVIDUOS SANOS
RESUMEN
Objetivo: Investigar la prevalencia de anticuerpos anti-fosfatidilserina (aFS) de tipo IgM, IgG e IgA en pacientes con síndrome antifosfolípido primario (SAFP) y en controles sanos; analizar la sensibilidad y la especificidad de los anticuerpos aFS para el diagnóstico de aFS y finalmente, evaluar las asociaciones entre los anticuerpos específicos aFS y las manifestaciones clínicas del SAF.
Métodos: Estudio transversal de 36 pacientes mujeres con SAFP y 200 donantes de san-gre. Se determinaron anticuerpos antifosfatidilserina de tipo IgM, IgG e IgA en pacientes y controles con SAFP utilizando una técnica propia y un kit comercial. A los pacientes con SAFP también se les determinó el anticoagulante lúpico (ACL), los anticuerpos anticardiolipina IgM e IgG (aCL), y los anticuerpos anti-β2 glucoproteína I (anti-β2GPI).
Resultados: La prevalencia de los anticuerpos AFS IgM, IgG, IgA en pacientes con SAFP fue la siguiente: 10,8-16,7%, 32,4-35,7%, y 16,1%, respectivamente. Aunque se encontró una sensibilidad relativamente baja para los anticuerpos AFS en el SAFP, la especificidad de los anticuerpos AFS IgM, IgG, IgA para el SAFP fue 94,7-98,9%, 95,3-96,3% y 97,9%, respectivamente. Todos los isotipos de AFS se asociaron significativamente con las manifestaciones obstétricas. Los anticuerpos AFS IgM se asociaron con un riesgo aumentado de trombosis venosa y arterial. Los anticuerpos AFS IgA se asociaron con la trombosis arterial mientras que los anticuerpos AFS IgG se asociaron con un mayor riesgo de eventos trombóticos venosos. Los anticuerpos AFS IgM e IgG se encuentran con frecuencia en asociación con anticuerpos anti-β2GPI.
Conclusiones: La prevalencia de anticuerpos AFS es baja en SAFS pero estos anticuerpos son altamente específicos para SAFP y se asocian con manifestaciones SAFP específicos.
Palabras claves: Síndrome Antifosfolípido, Anticuerpos Antifosfolípidos, Anticuerpos Antifosfatidilserina, Trombosis, Autoanticuerpos
INTRODUCTION
The antiphospholipid syndrome (APS) is characterized by venous, arterial or microvessel thrombosis and/or pregnancy morbidity in the presence of antiphospholipid (APL) antibodies. Manifestations such as thrombocytopenia, hemolytic anemia, heart valve disease, renal thrombotic microangiopathy, livedo reticularis among others are also considered as APS-related manifestations1,2. APL antibodies are a heterogeneous class of autoantibodies directed against anionic phospholipids, phospholipid-associated proteins and phospholipid-protein complexes (e.g., β2 glycoprotein I and prothrombin)3. To date, the APL antibodies recognized as APS markers for clinical classification purposes are lupus anticoagulant (LAC), anticardiolipin (aCL) antibodies, and anti-β2 glycoprotein I (anti-β2GPI) antibodies4-6. Nonetheless, the association between APS manifestations and other APL antibodies has been reported in the literature as well, including antibodies against phosphatidylserine (aPS) and against phosphatidylserine/prothrombin complex (aPS/PT)3,7.
Phosphatidylserine (PS) is an anionic phospholipid with a similar structure to cardiolipin except for the presence of a serine instead of a second glycerol group found in the cardiolipin molecule3. The pathogenic role of aPS antibodies has been highlighted in experimental studies with the development of APS features such as thrombocytopenia, prolonged activated partial thromboplastin time (aPTT), and increased rate of fetal resorption in mice immunized with IgG but not IgM aPS antibodies8,9. In the literature, a few studies have evaluated either antibodies against the complex aPS/PT in APS patients or antibodies against anionic PS in patients classified as primary APS (PAPS)10-14.
The aims of this study were to investigate the prevalence of IgM, IgG, and IgA aPS antibodies in patients with PAPS and in healthy controls; to analyze sensitivity, specificity of aPS antibodies for the diagnosis of APS; and to assess possible associations between aPS antibodies and specific APS manifestations.
MATERIALS AND METHODS
Study population
PAPS patients comprised 36 females with a mean age of 38.4 ± 11.8 years fulfilling the Sapporo criteria for APS.15 A total of 200 blood donors from São Paulo, Brazil were included after being considered healthy according to a clinical questionnaire to investigate cu-rrent or past autoimmune rheumatic disease, serious chronic infections, and neoplasia. All controls fulfilled the following inclusion criteria: 1) Age ≥ 18 years; 2) Negative serology for HIV, hepatitis B, and hepatitis C infection; 3) No regular use of medication. All samples used in this study were selected from a serum bank at Universidade Federal de São Paulo and all participants signed the consent form approved by the Institution‘s Ethics Committee.
Antiphospholipid antibodies
Enzyme-linked immunosorbent assays (ELISA) were performed according to the manufacturer’s operating instructions using commercial kits for the following: IgG and IgM aCL from DiaSorin (Saluggia, Italy) IgA aCL from Varelisa® (Phadia GmbH, Freiburg, Germany), IgM and IgG anti-β2GPI from the Binding Site (Birmingham, UK), and IgG and IgM aPS from Orgentec Diagnostika GmbH (Mainz, Germany). LAC was detected using aPTT from Diagnostica Stago, France and diluted Russel’s viper venon time (dRVVT) from Trinity Biotech, Wiclow, Ireland, according to the guidelines of the International Society of Thrombosis and Haemostasis16.
In addition, aCL (IgM and IgG), aPS (IgG, IgM, and IgA) and anti-β2GPI (IgG, IgM, and IgA) in house ELISAs were performed as previously described with some modifications.17,18 Briefly, the in house aCL ELISA test was performed with ELISA plates NUNC (Thermo Fisher Scientific Inc., Roskilde, Denmark) coated with bovine cardiolipin 50 µg/ml (Sigma-Aldrich, St. Louis, USA) and blocked with 10% adult bovine serum in saline at neutral pH for an hour. Plates were incubated overnight at 4°C with 50µl of serum diluted in PBS and 10% bovine serum albumin (BSA) at 1:50 dilution. Then, three washes were performed with PBS, and 50µl of conjugated anti-human IgG or IgM (Calbiochem, La Jolla, California, USA) was added to the plate. Then plates were incubated for 90 minutes at room temperature. After further washing steps, we added 50µl of developing solution and the plate was incubated at room temperature, protected from light for 30 minutes. The reading was performed with a spectrophotometer at a wavelength of 450 nanometers (nm). We constructed a calibration curve using international standards (Louisville APL Diagnostics Inc, Doraville United States, Prod # LAPL-GM100 IgG / IgM Calibrators). Values in optical density were interpolated this calibration curve and an equation was generated to provide results in units GPL and MPL.
The in house anti-β2GPI ELISA test was performed as follows: NUNC Maxisorp plates (Nalge Nunc International, Rochester, NY) were coated with 5μg/mL β2-glycoprotein I (Meridian Life Science, Inc. Memphis, USA) in PBS overnight at 2-8ºC. Plates were washed with 0.05% PBS-Tween 20 and 200µL of 0.5% PBS-BSA was added to wells and incubated during one hour at room temperature. After washing 3 times with 0.05% PBS-Tween 20, 50µL of serum dilutions representing six points of the standard curve (Louisville, APL Diagnostics, Inc. - Doraville, USA) and serum samples, all diluted 1:50 in 0.5% PBS-BSA were incubated for 1 hour at room temperature. After washing the plates 5 times with 0.05% PBS-Tween 20, wells were incubated with 100μL alkaline phosphatase–conjugated IgG, IgM or IgA goat anti–human antibodies (Sigma-Aldrich Co., St. Louis, USA) diluted 1:9000, 1:6000, and 1:1000, respectively in 0.5% PBS-BSA for 1 hour at 37ºC. Wells were then washed in 0.05% PBS-Tween 20 and incubated with 100μL tetramethylbenzidine (TMB) subs-trate (Siemens Healthcare, Marburg, Germany) for 20 minutes at room temperature and read at 450nm in a VictorTM X3 microplate spectrophotometer (PerkinElmer Inc, Massachusetts, USA).
For the in house anti-phosphatidilserine ELISA, SpetraPlateTM medium protein-binding plates (Perkin Elmer, Massachusetts, USA) were coated with 5μg/mL 1,2-Diacyl-sn-glycero-3-phospho-L-serine (Sigma-Aldrich Co., St. Louis, USA) in ethanol PA overnight at 2-8°C. Next, the plates were blocked with PBS containing 30% BSA for two hours at room temperature. After washing 3 times with cold PBS, serum samples diluted 1:50 in PBS and 30% BSA were incubated for 1 hour at room temperature. After washing 5 times with cold PBS wells were incubated with 100μL alkaline phosphatase–conjugated IgG, IgM or IgA goat anti–human antibodies (Sigma-Aldrich Co., St. Louis, USA) diluted at 1:9000, 1:6000, and 1:1000, respectively in PBS and 30% BSA for 1 hour at 37ºC. Wells were then washed in cold PBS as before and incubated with 100μL tetramethylbenzidine (TMB) substrate (Siemens Healthcare, Marburg, Germany) for 20 minutes at room temperature and read at 450nm in a VictorTM X3 microplate spectrophotometer (PerkinElmer Inc, Massachusetts, USA). The cut-off value was established as the mean plus two standard deviations from 8 negative samples. Results were provided as the index obtained by dividing the test sample by the cut-off value. A positive result was considered if index was ≥1.0.
Statistical analysis
Statistical analysis was carried out with the SPSS software (SPSS Inc, PASW Statistics for Windows, Version 18.0, Chicago, USA). Numerical data were displayed as mean, median, standard deviation, interquartile range or 95% confidence interval (95% CI) as appropriate, while categorical data were presented as total number and percentage. Analysis for significant differences between groups were performed using the Chi square test for categorical variables, while associations between specific isotypes of aPS antibodies with an increased risk of thrombosis or obstetric manifestations of APS were assessed by univariate logistic regression analysis and results were displayed in odds ratio (OR) and 95% CI. All analysis for diagnostic performance and clinical associations regarding aPS antibodies were performed using the in house technique as a reference while for the associations between aPS and different aPL antibodies, using either commercial kits and in house technique were analyzed with the Kappa coefficient and Spearman correlation coefficient. Level of significance accepted was 5% (p< 0.05).
RESULTS
aPS antibodies in PAPS patients and in control subjects
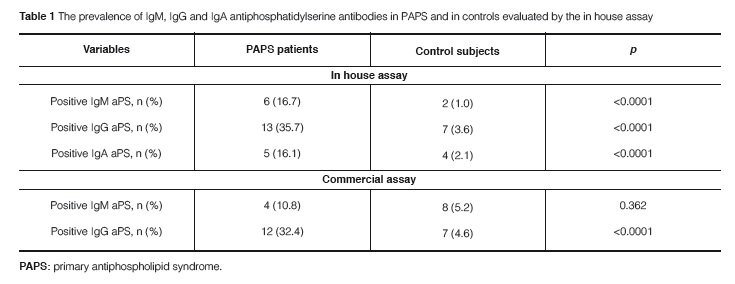
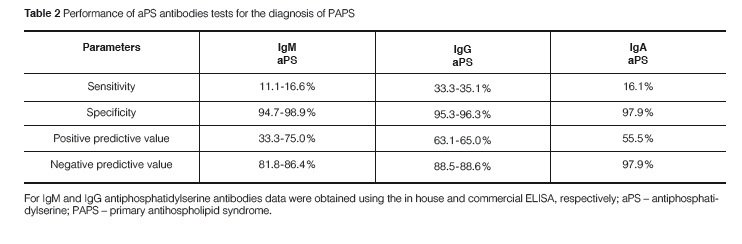
The positivity of any aPS antibody was 44.0% in PAPS patients and amongst them, 62.5% had more than one isotype of antibodies against PS. The prevalence of IgM, IgG, and IgA aPS antibodies was significantly higher in PAPS than in controls for most isotypes (Table 1). Even though a low sensitivity in the diagnosis of PAPS was observed for all aPS isotypes, the specificity, positive predictive value, and negative predic-tive value for the diagnosis of PAPS were quite high for both the in house technique or the commercial ELISA (Table 2). However, IgM aPS antibodies detected by the commercial ELISA kit yielded the lowest positive predictive value for PAPS.

Clinical features and aPS antibodies in PAPS patients
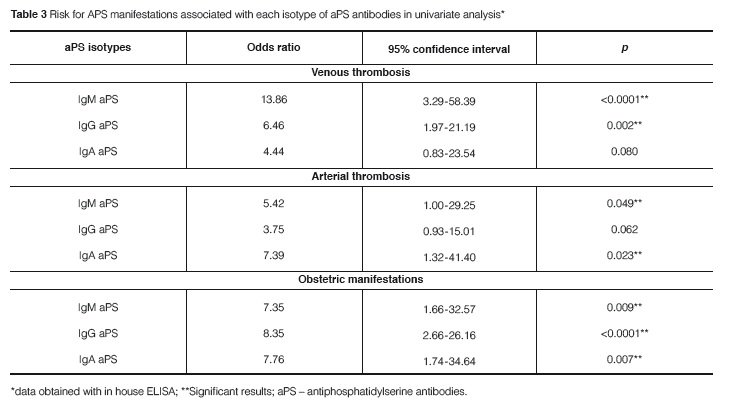
Thrombosis was the most common manifestation, being observed in 30 (83.3%) PAPS patients. Venous thrombosis was found in 16 (44.4%), arterial thrombosis in 13 (36.1%) and pulmonary embolism in 4 (11.1%). Ischemic stroke was the most common arterial thrombotic event, observed in 10 (27.8%) PAPS patients. Obstetric manifestations were found in 17 patients (47.2%) and they were the sole APS manifestation in 6 (36.6%) patients. According to univariate analysis all aPS isotypes were significantly associated with obstetric manifestations of APS. In contrast, the aPS isotypes behaved differently regarding the risk of thrombotic events. IgM aPS antibodies were strongly associated with an increased risk of venous thrombosis but they had a marginal association with arterial thrombosis. IgA aPS antibodies were associated with arterial thrombosis whereas IgG aPS antibodies were associated with an increased risk of venous thrombotic events (Table 3). No associations were found between disease manifestations and anti-β2GPI antibodies.
Association between aPS antibodies and other aPL antibodies
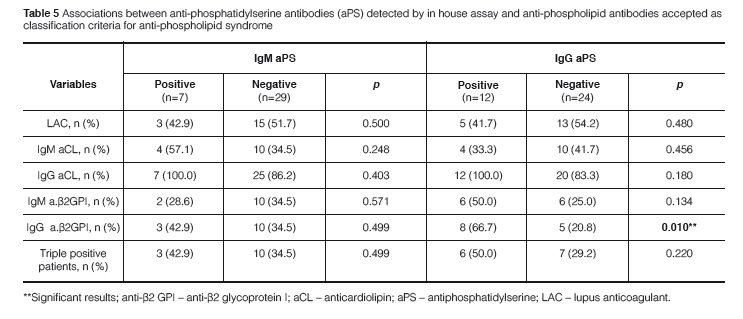
In a cross-sectional analysis, the prevalence of each aPL antibody test in PAPS patients varied accordingt o the use of in house assays and commercial kits, as follows: LAC 18 (50%), IgM aCL antibodies (5.6-27.8%), IgG aCL antibodies (33.3-38.9%), IgM anti-β2GPI (13.9-22.2%), IgG anti-β2GPI (13.9-27.8%), IgM aPS (13.9-19.4%), IgG aPS (33.3%) and IgA aPS (13.9%) (Table 4). In the longitudinal analysis of aCL tests repeated along time in PAPS patients, the prevalence of IgM and IgG aCL was 38.9% and 88.9%, respectively. The prevalence of triple positive PAPS patients (i.e. positivity of aCL, LAC, and anti-β2GPI) was 36.1% in this series.
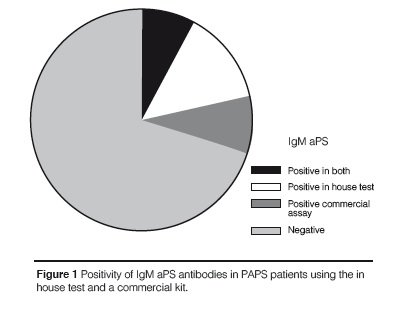
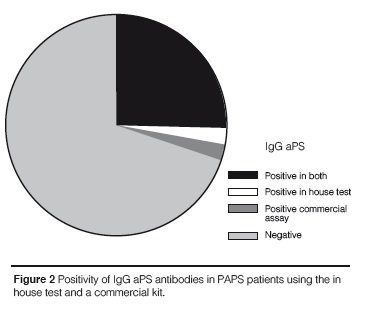
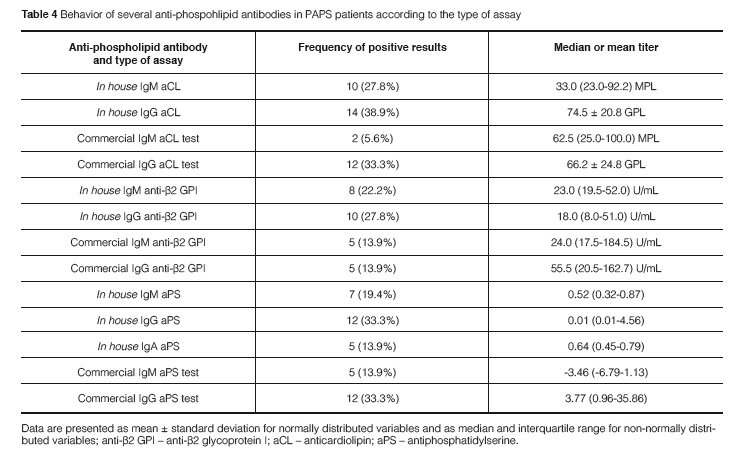
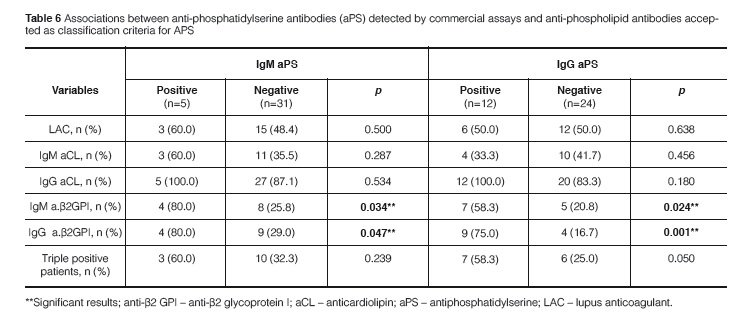
A strong agreement was found between the in house test and the commercial kit results for IgG aPS (Kappa coefficient = 0.875; p < 0.0001) and accordingly there was a strong correlation between titers of IgG aPS antibodies in the two ELISA systems (ρ = 0.763; p < 0.0001). Eleven out of 12 patients had positive results for IgG aPS antibodies when using the in house test and the commercial kit while only 2 out of 12 patients with IgM aPS antibodies were positive in both methods (Figures 1 and 2). In contrast there was no significant correlation between the in house test and the co-mmercial kit for IgM aPS (kappa coefficient = 0.204; p = 0.211) and accordingly there was no co-rrelation between the titers of IgM aPS in the two ELISA systems. An association between IgG aPS and IgG anti-β2GPI antibodies was found using the in house assay for aPS antibodies (Table 5) and their titers were significantly correlated as well (ρ = 0.675; p = 0.001). No association was found between IgM or IgA aPS antibodies determined with the in house method and other tests for aPL antibodies. Nonetheless, using commercial kits for aPS, we observed that IgM and IgG aPS were associated with both IgM and IgG anti- β2GPI (Table 6).
DISCUSSION
In this study, we have evaluated the role of aPS antibodies in the diagnosis of PAPS and the associations between IgM, IgG and IgA aPS antibodies with APS manifestations and other aPL antibodies. Although all isotypes of aPS antibodies had a low sensitivity, they were all highly specific for the diagnosis of PAPS. Moreover, IgM aPS antibodies were associated with an increased risk of venous and arterial thrombotic events while IgG aPS antibodies were associated with venous thrombosis and IgA aPS antibodies with arterial thrombosis. All isotypes of aPS antibodies were associated with obstetric manifestations of PAPS. Results obtained from the in house technique and from a commercial assay kit were very similar for IgG aPS antibodies while we could not find any significant agreement for IgM aPS. An association between aPS antibodies and anti-β2GPI antibodies was observed as well.
We found a prevalence of aPS antibodies lower than previously described for IgM aPS (50.0-77.0%) and IgG aPS (45.4-60.0%) in PAPS patients13,14. Similarly to previous studies that included Brazilian patients with PAPS19-21, the most prevalent aPL antibodies tests in this study were LAC and IgG aCL, and that might indicate a higher burden of these aPL antibodies rather than aPS antibodies in Brazilian patients with PAPS. Among controls evaluated in this study, the prevalence of aPS was similar to the 2% described for both IgG and IgM aPS13. The frequency of IgA aPS antibodies was similar to IgM aPS in the present series of PAPS patients whereas Radway-Bright et al could not detect IgA aPS in PAPS patients, but only in patients with systemic lupus erythematosus (SLE) (4%) and in APS associated with SLE (12%)13. The detection of aPS antibodies may not be regarded as a routinely useful diagnostic tool for APS due its low sensitivity. However, due to its high specificity for PAPS, the detection of aPS antibodies could be useful in patients with the so-called seronegative APS, in other words testing for aPS antibodies would only be worth when all other aPL antibodies are repetitively negative in a patients with suspicious APS.
Associations between aPS antibodies and specific manifestations of APS have been observed in several studies. Arterial thrombosis is a severe manifestation of APS and usually affects the central nervous system occurring as stroke or transient ischemic attacks1,2. We found a significant association between IgM and IgA isotypes of aPS antibodies with arterial thrombosis in PAPS patients while a trend for an association was found with IgG aPS antibodies. Controversial results regarding the association of aPS isotypes with stroke have been found in literature. Two cross-sectional studies have evaluated aPS antibodies with specific APS manifestations. Lopez et al found an increased risk of arterial thrombosis in APS patients presenting IgM (OR: 13.8; 95% CI: 2.9-63.9; p < 0.001) or IgG (OR: 35.8; 95% CI: 4.5-282.7; p < 0.001) aPS antibodies but not for IgA aPS. In contrast, Mucial et al found only an association between IgM aPS antibodies with thrombocytopenia.14,22 In assorted patients presenting stroke without a previous diagnosis of APS, a higher prevalence of IgG aPS antibodies was found in comparison with control subjects (57.7% vs. 4.8%, p < 0.001)23. In addition, Roggenbuck et al observed an association between IgM aPS and arterial thrombosis in a longitudinal study that included 223 follow-up samples of 45 APS patients along ten years24.
In this study, we could observe an association between IgM aPS antibodies
with venous thrombosis but not with other isotypes of aPS. On the other hand,
Lopez et al found an increased risk of venous thrombosis with IgM (OR: 9.6; 95%
CI: 2.0-46.3; p = 0.003) and IgG (OR: 21.0; 95% CI: 2.6-170.1; p < 0.001)
but not with the IgA isotype aPS antibodies in APS patients.22 However, no
significant association between aPS antibodies and venous thrombosis could be
seen a longitudinal study in APS.24 Regarding obstetric manifestations, all
isotypes of aPS antibodies were associated with an increased risk of pregnancy
morbidity in PAPS in the present series and similar OR values were observed among
the three isotypes. In the literature, the
association between aPS antibodies and obstetric
manifestations of has been strong for IgG aPS. In fact these autoantibodies may
be the sole positive test found in women with negative tests for other aPL antibodies24-26.
Standardization of aPL antibodies tests has been an issue for the diagnosis of aPS and that may account for the heterogeneous results in different reports27. In the present study some differences could be observed in results obtained by the use of in house assays and commercial kits for aCL, anti-β2GPI, and IgM aPS antibodies. However, good correlations regarding the positivity and OD values were observed between the in house and the commercial assay for IgG aPS antibodies.
This is the first time that an association is demonstrated between IgM and IgG aPS antibodies with IgM and IgG anti-β2GPI antibodies, especially when using the commercial kit for aPS. Previously, IgM and IgG aPS had been found in association with IgM and IgG aCL antibodies, respectively14. Thus both aPL antibodies may account together for the increased risk observed for APS manifestations with aPS antibodies. The lack of association between aPS antibodies and triple positive patients may indicate that the presence of aPS antibodies is not necessarily connected to an excessive multiproduction of aPL antibodies.
In conclusion, aPS antibodies seem to display low sensitivity and high specificity for the classification of PAPS. IgM aPS antibodies were associated with an increased risk of arterial and venous thrombotic events, while IgG aPS antibodies were associated with venous thrombosis, and IgA aPS antibodies were associated with arterial events. All aPS isotypes were shown to be a risk factor for obstetric manifestations of APS. IgG and IgM aPS antibodies were associated with IgM and IgG anti-β2GPI antibodies in PAPS patients. The detection of aPS antibodies does not seem to present any advantage over other APL antibody tests and may be of value in patients with suspicious APS without positive APL tests.
REFERENCES
(1) Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet. 2010;376(9751):1498-1509.
(2) L evine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N E ng J Med. 2002;346(10):752-63.
(3) Shoenfeld Y, Twig G, Katz U, Sherer Y. Autoantibody explosion in antiphospholipid syndrome. J Autoimmun. 2008;30(1-2):74-83.
(4) G alli M, Luciani D, Bertolini G, Bardui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood. 2003;101(5):1827-32.
(5) O patrny L, David M, Khan SR, Shrier I, Rey E. Association between antiphospholipid antibodies and recurrent fetal loss in women without autoimmune diseases: a metaanalysis. J Rheumatol. 2006;33(11):2214-21.
(6) Galli M, Borrelli G, Jacobsen EM, Marfisi RM, Finazzi G, Marchiori R, et al. Clinical significance of different antiphospholipid antibodies in the WAPS (warfarin in the antiphospholipid syndrome) study. Blood. 2007;110(4):1178-83.
(7) Alessandri C, Conti F, Pendolino M, Mancini R, Valesini G. New autoantigens in the antiphospholipid syndrome. Autoimmun Rev. 2011;10(10):609-16.
(8) Blank M, Tincani A, Shoenfeld Y. Induction of experimental antiphospholipid syndrome in naïve mice with purified IgG antiphosphatidylserine antibodies. J Rheumatol. 1994;21(1):100-4.
(9) Yodfat O, Blank M, Krause I, Shoenfeld Y. The pathogenic role of anti-phosphatidylserine antibodies: active immunization with the antibodies lead to the induction of the antiphospholipid syndrome. Clin Immunol Immunopathol. 1996;78(1):14-20.
(10) Atsumi T, Ieko M, Bertolaccini ML, Ichikawa K, Tsutsumi A, Matsuura E, et al. Association of autoantibodies against the phosphatidylserine-prothrombin complex with manifestations of the antiphospholipid syndrome and with the presence of lupus anticoagulant. Arthritis Rheum. 2000;43(9):1982-93.
(11) Hoxha A, Ruffatti A, Tonello M, Bontadi A, Salvan E, Banzato A, et al. Antiphosphatidylserine/prothrombin antibodies in primary antiphospholipid syndrome. Lupus. 2012;21(7):787-9.
(12) Vlagea AD, Gil A, Cuesta MV, Arribas F, Diez J, Lavilla P, et al. Antiphosphatidylserine/prothrombin antibodies (aPS/PT) as potential markers of antiphospholipid syndrome. Clin Appl Thromb Haemost. 2012. Available from ttp://cat.sagepub.com/content/early/2012/02/27/1076029612437578.long [Accessed on September 17th 2012].
(13) Radway-Bright E, Ravirajan CT, Isenberg DA. The prevalence of antibodies to anionic phospholipids in patients with the primary antiphospholipid syndrome, systemic lupus erythematosus and their relatives and spouses. Rheumatology (Oxford). 2000;39(4):427-31.
(14) Musial J, Swadzba J, Motyl A, Iwaniec T. Clinical significance of antiphospholipid protein antibodies. Receiver operating characteristics plot analysis. J Rheumatol. 2003;30(4):723-30. 15. Wilson WA, Gharavi AE, Koike T, Lockshin MD, Branch DW, Piette JC, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42(7):1309-11.
(16) Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2009;7(10):1737-40.
(17) G haravi AE, Harris EN, Asherson RA, Hughes GR. Anticardiolipin antibodies: isotype distribution and phospholipid specificity. Ann Rheum Dis. 1987;46(1):1-6.
(18) Viard JP, Amoura Z, Bach JF. Anti-b2-glycoprotein I antibodies and thrombosis. Clin Rev Allergy Immunol. 1995;13(1):67-72.
(19) De Souza AW, Silva NP, de Carvalho JF, D’Almeida V, Noguti MA, Sato EI. Impact of hypertension and hyperhomocysteinemia on arterial thrombosis in primary antiphospholipid syndrome. Lupus. 2007;16(10):782-7.
(20). Rodrigues CE, Bonfá E, Caleiro MT, Vendramini MB, Bueno C, L opes JB, et al. Coexistance of metabolic syndrome and primary antiphospholipid syndrome is associated with arterial events. Arthritis Care Res (Hoboken). 2012; 64(10):1576-83.
(21) de Souza AW, Keusseyan SP, da Silva NP, Sato EI, Andrade LE. Antinucleosome antibodies and primary antiphospholipid syndrome: na observational study. Rev Bras Reumatol. 2012; 52:357-65.
(22) L opes LR, Dier KJ, Lopez D, Merrill JT, Fink CA. Anti-beta 2-glycoprotein I and antiphosphatidylserine antibodies are predictors of arterial thrombosis in patients with antiphospholipid syndrome. Am J Clin Pathol. 2004; 121(1):142-9.
(23) Kahles T, Humpich M, Steinmetz H, Sitzer M, Lindhoff-Last E. Phosphatidylserine IgG and beta-2-glycoprotein I IgA antibodies may be a risk factor for ischemic stroke. Rheumatology. (Oxford) 2005;44(9):1161-5.
(24) Roggenbuck D, Egerer K, Feist E, Burmester GR, Döner T. Antiphospholipid antibody profiling: associations with the clinical phenotype of antiphospholipid syndrome? Arthritis Rheum. 2012;64(8):2807-8.
(25) Velayuthaprabhu S, Archunan G. Evaluation of anticardiolipin antibodies and antiphosphatidylserine antibodies in women with recurrent abortion. Indian J Med Sci. 2005; 59(8):347-52.
(26) Silver RM, Pierangeli SS, Edwin SS, Umar F, Harris EN, Scott JR, et al. Pathogenic antibodies in women with obstetric features of antiphospholipid syndrome who have negative test results for lupus anticoagulant and anticardiolipin antibodies. Am J Obstet G ynecol. 1997;176(3):628-33.
(27) Devreese KM. Standardization of antiphospholipid antibody assays. Where do we stand? Lupus. 2012;21(7):718-21.
Fecha de envío: 15/11/2015 - Fecha de aprobación: 01/12/2015